Tirzepatide
research use only; not for human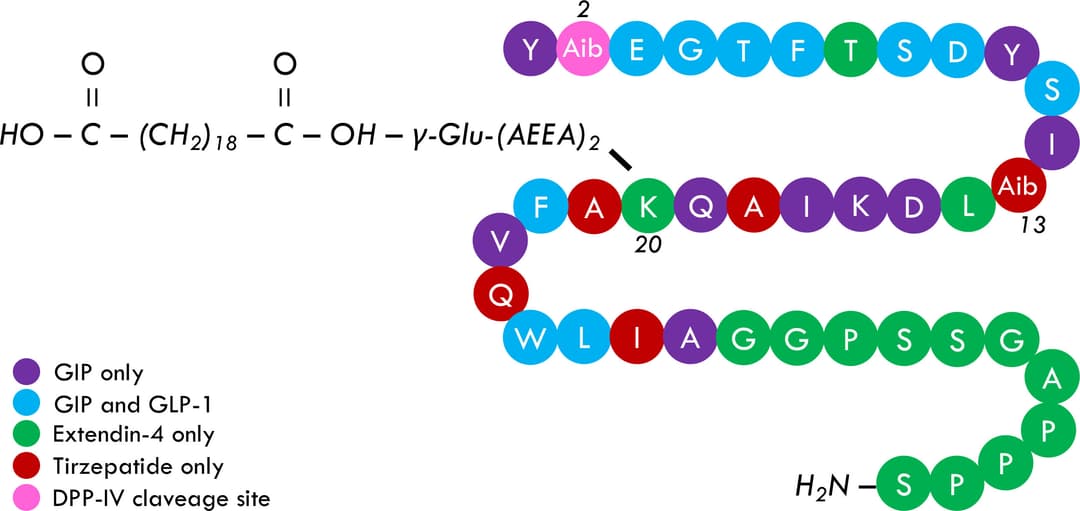
Description
Tirzepatide, also known as LY 3298176, is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. Tirzepatide has a greater affinity to GIP receptors than to GLP-1 receptors, and this dual agonist behavior has been shown to produce greater reductions of hyperglycemia compared to a selective GLP-1 receptor agonist. Signaling studies reported that tirzepatide mimics the actions of natural GIP at the GIP receptor. Tirzepatide was approved for improving blood sugar control in adults with type 2 diabetes, as an addition to diet and exercise.
Mechanism of Action
Tirzepatide is an investigational drug that is being developed for the treatment of type 2 diabetes. It is a dual-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) and glucose-dependent insulinotropic polypeptide receptor agonist (GIPRA) compound. The mechanism of action of tirzepatide is based on its dual agonism of both the GLP-1 and GIP receptors, which leads to improved glucose control and reduction in body weight. By stimulating the GLP-1 receptor, tirzepatide enhances insulin secretion in a glucose-dependent manner, slows gastric emptying, and reduces food intake, leading to improved glycemic control. By stimulating the GIP receptor, tirzepatide enhances glucose-dependent insulin secretion, which contributes to improved glucose control.